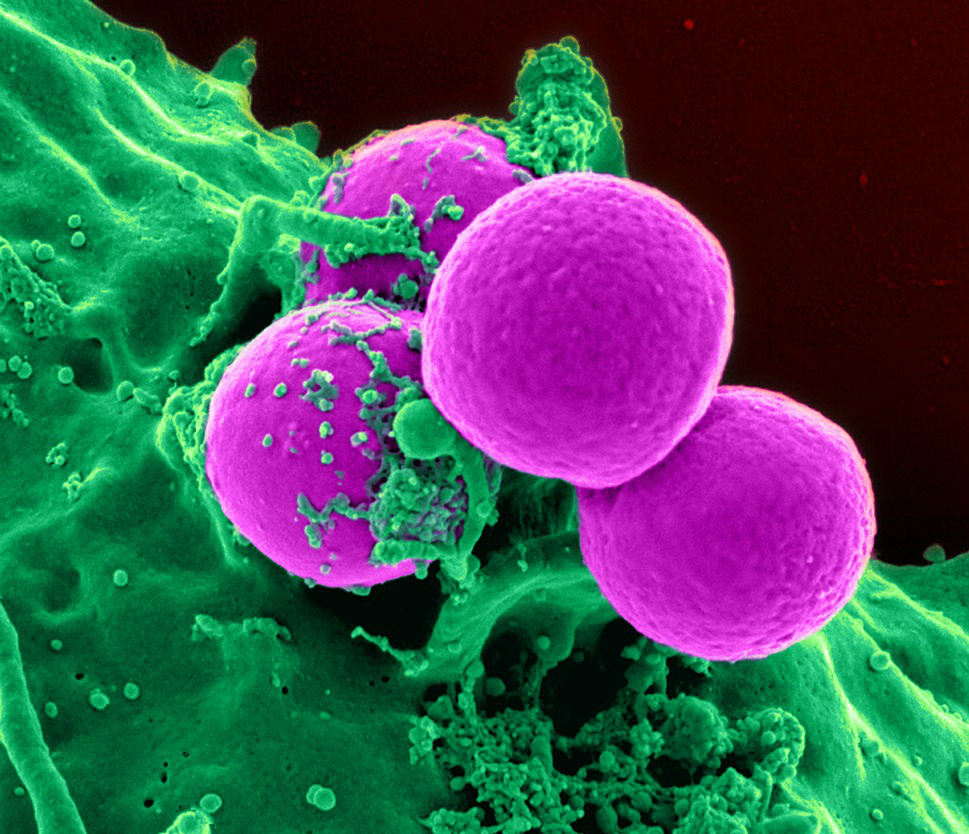
Related Posts

This report shall seek to explore the pharmacokinetics, pharmacodynamics, efficacy and side effects of the commonly prescribed drug Ibuprofen, the mode of action of turmeric and glucosamine and the potential interaction between the two nutrients and ibuprofen. The assignment shall include a section where the mode of action of the drug and nutrients will be […]

Sometimes the energy feels dense, as if two oceans have suddenly collided. And sometimes we can feel the weight of the collective, bringing up unconscious patterns or new awareness. Either way, if it feels thick, then it’s likely that layers of burdened energies are rising, being cleared, and making space for something new. Many of […]

Embarking on a journey of personal transformation through healing techniques is something central to most of us… Cultivating positive change and growth in our lives is a full time job – and we need all the support we can get. Especially from those teachers and guides who bring together various strands in an integrative manner […]


 The majority of animal studies and the few human studies that exist suggest ibuprofen inhibits or delays fracture healing (Cottrell and O’Connor 2010) as PG2 is used to stimulate vascular changes, bone absorption, proliferation of ostergenitors, chondrogenesis, chondrolysis, bone formation and metabolism (Chang et al 2000). However, in a placebo-controlled study that compared ibuprofen to rofecoxib, a longer-term use therapeutic NSAID for rabbit fibula osteomy healing, ibuprofen treatment appeared to delay bone healing however rofecoxib treatment produced worse fracture healing likely due to continuous COX-2 inhibition rather than cyclical COX-2 inhibition with ibuprofen (O’Connor et al 2009). On the whole, these points count against the analgesic efficacy of ibuprofen in bone healing and alternatives should be considered.
The majority of animal studies and the few human studies that exist suggest ibuprofen inhibits or delays fracture healing (Cottrell and O’Connor 2010) as PG2 is used to stimulate vascular changes, bone absorption, proliferation of ostergenitors, chondrogenesis, chondrolysis, bone formation and metabolism (Chang et al 2000). However, in a placebo-controlled study that compared ibuprofen to rofecoxib, a longer-term use therapeutic NSAID for rabbit fibula osteomy healing, ibuprofen treatment appeared to delay bone healing however rofecoxib treatment produced worse fracture healing likely due to continuous COX-2 inhibition rather than cyclical COX-2 inhibition with ibuprofen (O’Connor et al 2009). On the whole, these points count against the analgesic efficacy of ibuprofen in bone healing and alternatives should be considered.